And it is also used it food processing. Beta-decay is divided into two forms beta decay positive and beta decay negative which emits positrons and electrons respectively.

12 Alpha Decay Examples In Real Life Studiousguy
The best known everyday application of alpha decay is the smoke detector.

. There are a number of applications where we use the concept of radioactive decay in real life some of them are listed below. The radioisotopes in smoke detectors emit alpha particles. A smoke detector consists of two metal plates with a small space between them.
One of the prominent applications of alpha decay can be observed in the smoke detectors installed in buildings. Radioisotopes are radioactive isotopes or isotopes that emit charged particles and energy from the nucleus. Write a description of alpha decay for Po-211 and in your own words define half life based upon what you observe on the timeline at the top of the simulation Complete the following alpha decay equations using Lesson 7 Chap 11 pt1 on Sakai a resource.
How is alpha decay used in everyday life. Inside the smoke detertor alpha particles are released. Alpha radiation is also used to power a wide array of seismic and other oceanographic devices.
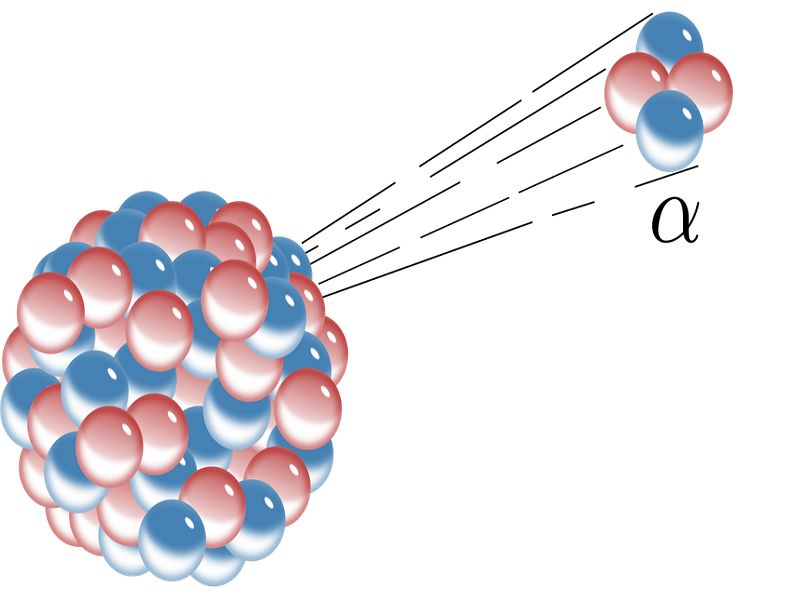
The following are some of the real-world applications of alpha decay. Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle helium nucleus and thereby transforms or decays into a different atomic nucleus with a mass number that is reduced by four and an atomic number that is reduced by two. How is alpha decay used in everyday life.
Watch alpha particles escape from a polonium nucleus causing radioactive alpha decay. Want to read all 3. Alpha decay is safer than other emissions.
The radioactive materials produce a lot of heat during the decay process where the alpha radiation is converted into thermal energy due to the movement of the atoms in the element. Alpha decay the emission of helium ions exhibits sharp line spectra when spectroscopic measurements of the alpha-particle energies are made. Some chemical change examples in our everyday life are mentioned below.
Alpha particles explain how a smoke detector works. An alpha particle is tiny particle consisting of two protons and two neutrons. Refer to Wikipedia and give at least two uses The Americium-241 in smoke detectors releases alpha particles and when smoke particles catch them the alarm is set off.
Give at least two uses _____ _____ Investigating Half-Life of Alpha Decay. These plates have wires connected to a battery and current monitor. 411 Z A X Z 2 A 4 X 2 4 He.
Radioactive elements that undergo alpha decay are used in smoke detectors see Figure 3. This in turn ionizes the air inside the detector. Smoke in the detector absorbs this alpha radiation so if smoke is present the ionization is altered and the.
However if alpha-emitting materials are taken into the body by breathing eating or drinking they can expose internal tissues directly and may therefore cause biological damage. Alpha decay or α-decay is a type of radioactive decay in which the atomic nucleus emits an alpha particle thereby transforming or decaying into a new atomic nucleus. They are a safe power source radioisotopes and space probes.
It can be written symbolically as. An alpha particle is identical to the nucleus of a helium-4 atom which consists of two protons and two. Here the atomic mass number of the newly formed atom will be reduced by four and the atomic number will be reduced by two.
Writing and balalncing nuclear reactions videos 2. Oct 25 2015. How is alpha decay used in everyday life.
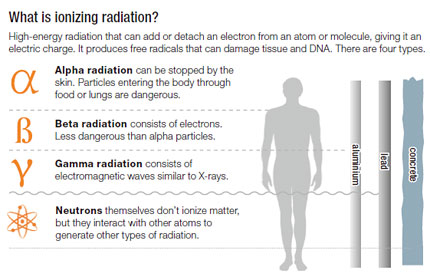
Strontium-90 is the most common material used in these alpha decay batteries. Alpha radiation can also kill cancer cells. Beta radiation consists of electrons.
Alpha decay is observed for the elements heavier than lead and for a few nuclei as light as the lanthanide elements. See how random decay times relate to the half life. These unmanned devices are often located in isolated locations such as on the ocean floor which limits the practicality of short-term batteries.
The definition of Thermal energy is twofold Energy and Thermal - Energy can be defined as the ability to work which introduces another word work Work is the movement The process in nuclear physics in which the nucleus of an atom splits into two daughter nuclei. Alpha radiation can be stopped completely by a sheet of paper or by the thin surface layer of our skin epidermis. Any smoke particles that enter the unit reduce the current and set off an alarm.
Beta rays with 05v energy are a distance of 1 M in the atmosphere and the range depends on the power of the particle. To see how watch this video. For eveneven alpha emitters the most intense alpha group or line is always that leading to the ground state of the daughter.
One of the plates contains a small amount of the radioactive element americium which gives off alpha particles. Weaker lines of lower energy go to excited states and there are frequently numerous lines observable. Alpha particles emitted by the americium ionize the air making the air conductive.
We use X to indicate any element defined by its nuclear charge Z and Z -2 in this equation. It can cause cell mutation leading to diseases such as cancer. Smoke detectors typically make use of a radioactive element known as americium-241.
Americium is one frequently used element as it is a major alpha particle source. In the same manner it can also be used to cure cancer - when a large dose of radiation emitting waves are directed to cancerous cell. Course Hero member to access this document.
They are used in smoke detectors. The heat energy produced is used in various. Upload your study docs or become a.
Despite the fact that these devices save lives the question are smoke detectors safe.
0 Comments